It was created by Alex Brinley Charis Conwell and Siena Joy for our AP Chemistry class. The features they have suit the activities they may be used for.

Designing A Hand Warmer Chm 11100 General Chemistry Purdue Studocu
This is a video outlining a Flinn Lab on designing a hand warmer.

. Think of an outdoor activity you enjoy where a hand warmer may. These were our amazing recommended products on the list of perfect designing a hand warmer pre lab answers you can get online. Chemistry 1 Final Exam Review - Definition Notes.
Conclusion - Hand warmer challenge. Sodium hypochlorite in bleach lab write up. The Designing of a Hand Warmer Lab 1192021 I.
The hand warmer is struck in a manner that ruptures the inner pouch releasing the ionic salt into the water of the outer pouch. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. When chromium chloride CrCl 2 is dissolved in water the temperature of the water decreases.
But disposable hand warmers. 1 Measure out 2 separate samples of 1000 mL of distilled water. Hand warmers work because of a rusting process.
1 Place an opened Ziploc bag into a beaker and fold the edges over the side of the beaker so that you will be able to pour reactants. Designing a Hand Warmer. This lab will require you to put your chemistry skills to commercial use.
Qhand warmer qwater qsodium acetate or ΔH solmsodium acetate mCΔTwater mCΔTsodium acetate 3. 2 Heat one to about 50C and place other one in calorimeter at around 20C 3 Add heater water to calorimeter cover top wait 15 seconds measure temp. They all have different features for example some stay hot for a long time some only stay hot for a short time.
Designing a Hand Warmer By. P calorimetry 25 lab report. Devon Atchison Tyler Pennington and Vicki Smith Observations Immediately after putting in the Lithium Chloride a steam or a white powder arising from the solution was observed.
Heat of solution for an ionic compound. Energyenthalpy change with process of dissolving a solute in solvent. Is the heat of solution exothermic or endothermic.
Purpose The Purpose of this advanced inquiry lab is to design an effective hand warmer that is inexpensive nontoxic and safe for the environment. The filling process seemed pretty cumbersome and the warmer â DOLORES GENDE. We calculated the different amount of heat that was being released on each.
Designing an Effective Hand Warmer. Designing a Hand Warmer Purpose. The backbone of these applications is.
Does the temperature of the hand warmer remain constant increase or decrease after they are activated. To determine the best solute to use to make a safe hand warmer. Designing a Hand Warmer Lab Introduction.
As you wish the hard-earned money should spend wisely we have been working for a long time on this list with extensive research and review to help you get the perfect product in between your hands. Water Heater Questions F2021 water heater questions. Zippo Hand Warmer Black Matte.
Safety Being able to carefully handle all ionic solids and chemicals and being safe with the glass used and any measurement tools used to collect data. LAB EXPERIMENTS apphysicsb. Sandra Bergh Created Date.
And safety information to propose a design for the best all-around hand warmer. The temperature increases as heat is released by the solidification process. Looking at the results we saw which chemicals created more amount of heat when its being applied to water and salt.
An ideal hand warmer increases in temperature by 20 C but no. Include Designing a Hand Warmer Thursday November 17 2016 1028 AM Lab Reports Page 1. The stated purpose of this lab which is to design an effective hand warmer that is expensive non-toxic and safe for the environment was met.
IMF Lab report - Grade. From instant cold packs to flameless ration heaters and hand warmers the energy changes accompanying physical and chemical transformations have many consumer applications. 4Fes 3O 2 g - 2Fe 2 O 3 s In todays lab we will explore how the rusting process is used in hand warmers.
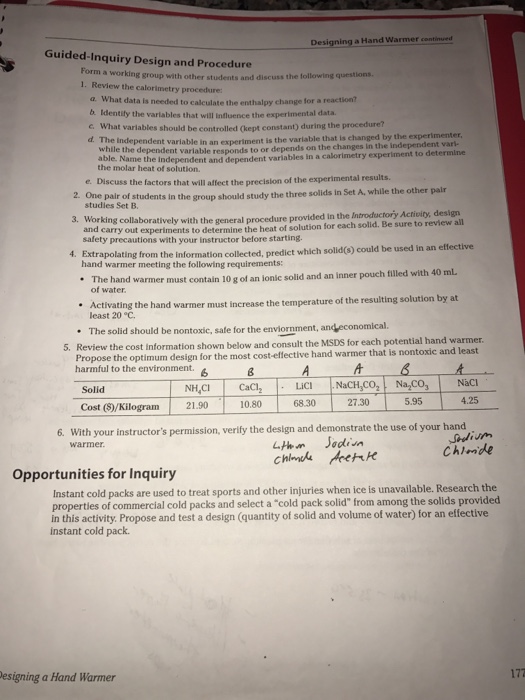
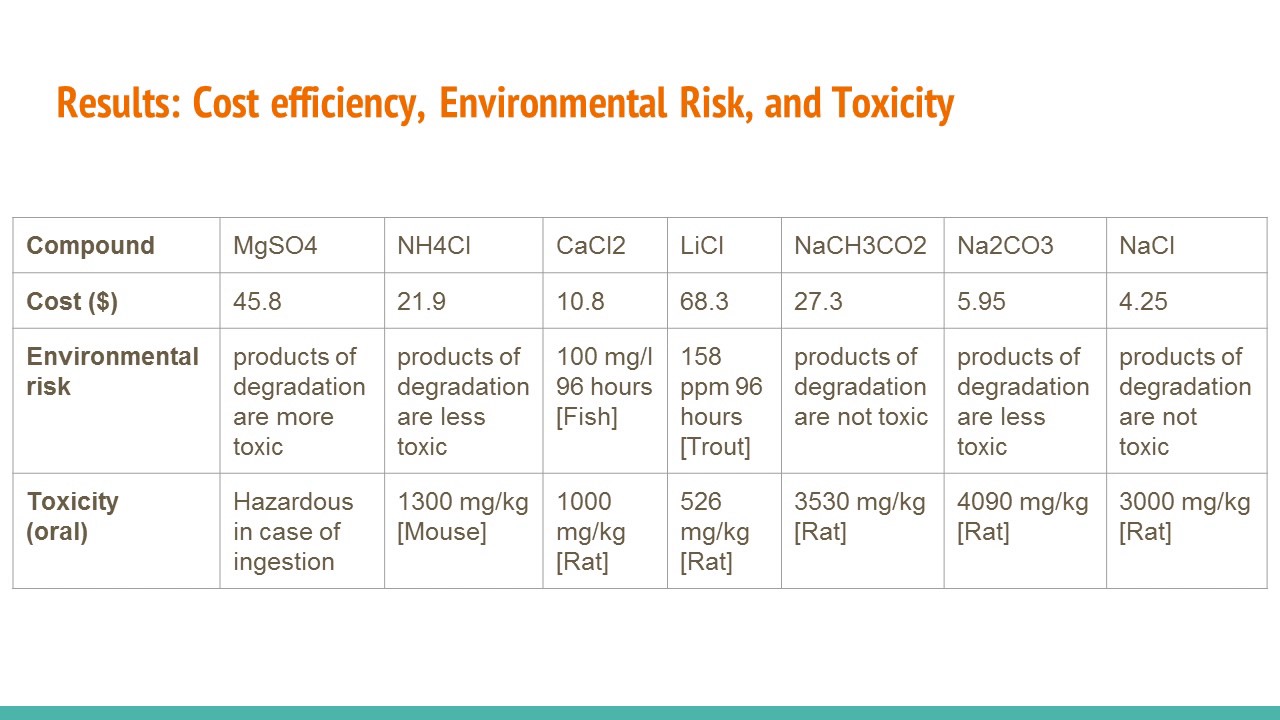
Microsoft Word - Grading Rubric-Designing Hand Warmer Labdocx Author. Which is strongerthe attractive forces between water molecules and chromium and chloride ions or the combined. CaCl2 Calcium Chloride MgSO4 Magnesium Sulfate and LiCl Lithium Chloride.
When some ionic salts are dissolved in water the reaction is exothermic. In the experiment we used three types of Chemicals. Lab 9 Data - Lab assignment for CHEM105a.
The rusting is a redox reaction and the equation is as follows. During the lab six different solids three different chemicals for each group along with their costs and individual Safety Data Sheets. Designing the Ideal Hand Warmer.
Using Prezi Video for virtual sales presentations that convert. I carried out this experiment and calculated the molar heat of solution for my salts and used the MSDS to determine the most viable and effective hand warmer. How to schedule fewer meetings and get more done.
The process of kinetic energy transfer at the particulate scale is referred to in this course as heat transfer and the spontaneous direction of the transfer is always from a. Hand warmers are small packets that people put inside gloves or mittens on cold days to keep their fingers warm. Heat of Solution measures.
Designing a Hand Warmer Lab Report November 26th 2017 Period 4 I. Purpose The purpose of this lab is to design an effective hand warmer that is inexpensive effective nontoxic and safe for the environment. Table Part B and C.
Write a thorough paragraph this will be your WAC this month discussing the criteria for the hand warmer design and why you are choosing one over the other. They are very popular with people who work outside in winter or who do winter sports. Measure heat transfer using a calorimetry investigate energy changes accompanying the formation of substances and design a hand warmer that is reliable safe and inexpensive.
You have seen how some hand warmers work. 7 Calculate the molar heat of solution q solution. 13- calculate calorimeter constant Part B and C.
Chem 100 Exam 1 Study Guide w answers. The salt dissolves and the.
Solved Designing A Hand Warmer Continued Kn Chemistry Review Chegg Com

Designing A Hand Warmer By Makayla Sabo
Designing A Hand Warmer Lab Youtube

Designing A Hand Warmer Chm 11100 General Chemistry Purdue Studocu

Ap Chemistry Files 4 Designing A Hand Warmer

Handwarmer Ap Chem Lab Report Alexis Salim Lab Group 5 03 February 2017 Handwarmer Lab Report I Purpose The Purpose Of This Lab Was To Test Course Hero

Hand Warmer Lab Pdf Designing A Hand Warmer Lab Abstract The Purpose Of This Lab Is To Design An Effective Hand Warmer That Is Inexpensive Nontoxic Course Hero

0 comments
Post a Comment